AsTher Process Calculator for MS Excel
8.4. Enthalpy-Concentration, Liquid-Gas-Concentration-Diagrams of Ethanol-Water Mixtures
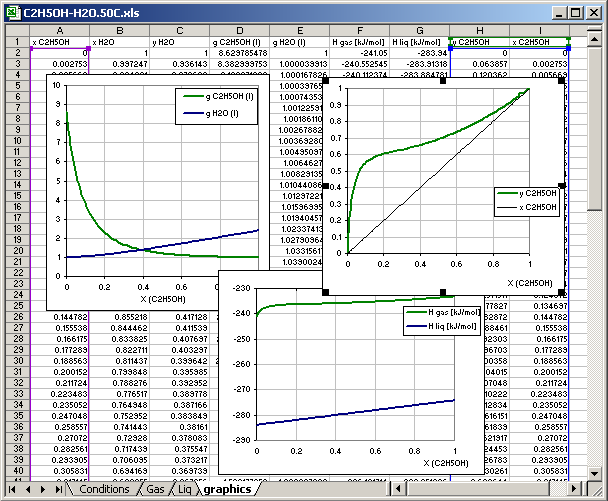
This example shows the sheets of the MS Excel file to plot state diagrams using the empirical equations of activity coefficients in the application AsTher Process Calculator.

Downlad fileC2H5OH-H2O.50C.xls
The C2H5OH-H2O.50C.xls contains 4 sheets: conditions, Gas, Liq and
graphics.
The data in the sheets are written in following
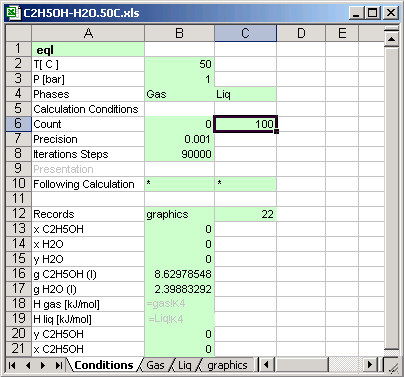
Cell [B2] Temperature of the thermodynamic system
Cell [B3] Pressure of the thermodynamic system
Cell [B6] Calculation is made 100 time, the input values changed in
depend of the calculation count for different concentrations of the substances.
Cell [B10] * : means if the calculation count is less than 100 [C6], then
the next calculations system is defined in the same file.
Cell [C10] * : means if the calculation count is less than 100 [C6], then the
next calculations system is defined in the same condition sheet.
Cell [B12] graphics: Calculations result is written in the sheet named graphics, the sheet must exist in the same file (or in the same MS Excel book)
Cells [B13] to [B21]: the cells contain links to another cell in different sheet, which data are recorded in the sheet graphics
8.4.2. Sheet for gas phase
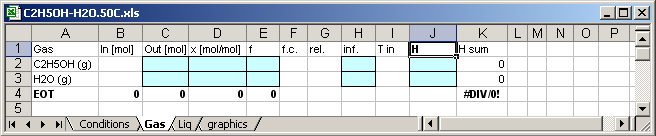
In cell [J1] written H, so that the application writes enthalpies
of the substances in the cells [J2] and [J3].
Enthalpy of the C2H5OH in the mixture [K2]=C2*J2
Enthalpy of the H2O in the mixture [K3]=C3*J3
Molar Enthapy of the mixture [K4]=(K2+K3)/C4
Mass of the mixture in mol [C4]=C3+C4
The calculation results are written in the blue coloured cells,
8.4.3. Sheet for liquid phase
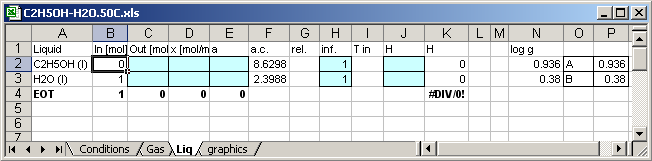
The cell [B2]=Conditions!B6*0.01 the input values are varied in depend
of the calculation count.
The cell [B2]=1-B2 the input values are varied in depend of the
calculation count.
Enthalpy of the C2H5OH in the mixture [K2]=C2*J2
Enthalpy of the H2O in the mixture [K3]=C3*J3
Molar Enthapy of the mixture [K4]=(K2+K3)/C4
Mass of the mixture in mol [C4]=C3+C4
In cell [J1] written H, so that the application writes enthalpies of the substances in the cells [J2] and [J3].
The activity coefficients of the binary mixtures given by Van Laar
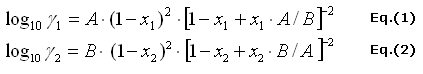
There are Eq.(1) the activity coefficients of ethanol and Eq.(2)
the activity coefficient of water in the binary mixture.
The constants A and B are given by C.A. Jones, A.P.
Schoenborn, Ind. Chem. Eng. 40 (1940) 1273-1276
for 50 C
A is in Cell [P2]=0.936
B is in Cell
[P3]=0.38
Eq.(1) and Eq.(2) written in Cells [N2] and [N3]
[N2]=P2*POWER(1-D2;2)*POWER((1-D2+D2*P2/P3);-2)
Eq. (1)
[N3]=P3*POWER(1-D3;2)*POWER((1-D3+D3*P3/P2);-2)
Eq. (2)
The cells [F2] and [F3] contain the activity coefficients:
[F2]=POWER(10;N2)
[F3]=POWER(10;N3)
8.4.4. Calculations
When Process Calculator for MS Excel runs, and button Calculation
is pressed, the calculations are carried out 100 time for different input
values. The Result of the calculation is written in the sheet graphics.
8.4.5. Sheet for Graphics
The sheet graphics contains no any data before the calculations.
The data in th colums A.. I are written by application corresponding the
parameters in the conditions sheet.
